
|
Blue fluorescent protein TagBFP
- Bright blue fluorescence
- Monomeric protein with successful performance in fusions
- Fast maturation, high photostability
- Extremely high pH-stability
- Recommended for protein labeling, acidic organelle labeling, FRET applications
 |
TagBFP (scientific name mTagBFP) is a monomeric blue fluorescent protein generated by site-specific and random mutagenesis of TagRFP [Subach et al., 2008]. TagBFP possesses bright blue fluorescence with excitation/emission maxima at 402 and 457 nm, characterized by high photostability and extremely high pH-stability.
Compared to EBFP2 [Ai et al., 2007], TagBFP is more then 1.8 times brighter, much more pH-stable and has twice shorter maturation half-time at 37°C. Narrow fluorescence emission peak of TagBFP provides for accurate and easy spectral separation with cyan and green fluorescent proteins and makes it a preferable tag for multicolor labeling.
Good overlap between the emission spectrum of TagBFP and the absorbance spectra of TagGFP2 allows using these two proteins as a FRET pair.
|
Main properties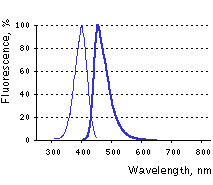
TagBFP normalized excitation (thin line) and emission (thick line) spectra.
Spectra viewer tool
Download TagBFP spectra (xls)
| | CHARACTERISTIC | |
|---|
* Brightness is a product of extinction coefficient and quantum yield, divided by 1000.
| | Molecular weight, kDa | 26 | | Polypeptide length, aa | 233 | | Fluorescence color | blue | | Excitation maximum, nm | 402 | | Emission maximum, nm | 457 | | Quantum yield | 0.63 | | Extinction coefficient, M-1cm-1 | 52 000 | | Brightness* | 32.8 | | Brightness, % of EGFP | 99 | | pKa | 2.7 | | Structure | monomer | | Aggregation | no | | Maturation rate at 37°C | fast | | Photostability | high | | Cell toxicity | not observed | | Main advantages | bright blue monomeric fluorescent protein | | Possible limitations | not observed |
|
|---|
Recommended filter sets and antibodies
TagBFP can be recognized using Anti-tRFP antibody (Cat.# AB233) available from Evrogen.
The protein can be detected using common fluorescence filter sets for BFP, DAPI, and other blue dyes.
Recommended filter sets are: XF119-2*, QMAX-Blue*, XF131, XF06, XF13-2, XF03, XF11, XF129-2, XF05-2 (Omega Optical); DAPI-5060B* and DAPI-1160A (Semrock); 31037, 31041, 31016*, 31021, 31000v2, 1009v2, 31013v2, 11005v2, 31047 (Chroma Technology Corp.).
* – preferred filter sets
Performance and use
TagBFP can be easily expressed and detected in a wide range of organisms. Mammalian cells transiently transfected with TagBFP expression vectors produce bright fluorescence in 10-12 hrs after transfection. No cytotoxic effects or visible protein aggregation are observed.
TagBFP performance in fusions has been demonstrated in the β-actin and α-tubulin models. It can be used in multicolor labeling applications with cyan, green, yellow, red, and far-red fluorescent dyes.
High pH-stability with pKa=2.7 makes it possible to use TagBFP for imaging in acidic organelles, such as late and recycling endosomes and lysosomes.
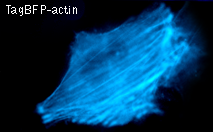 | 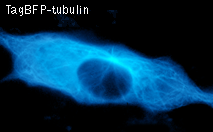 | HeLa cells expressing TagBFP fusion proteins.
|
Emission spectrum of TagBFP and absorbance spectra of GFPs demonstrate good overlap, suggesting TagBFP may be a good donor for GFP acceptor for Forster resonance energy transfer (FRET). Among available GFPs, TagGFP2 is prefferable because of the small absorbance below 400 nm where TagBFP is excited. High efficiency of TagBFP-TagGFP2 FRET pair was demonstrated in living cells [Subach et al., 2008].
Available variants and fusions
| Variant | Description | Related vector | Cat.# | Click for image |
|---|
 |
|
Humanized TagBFP
|
TagBFP codon usage is optimized for high expression in mammalian cells [Haas et al., 1996], but it can be successfully expressed in many other heterological systems.
|
pTagBFP-C
|
FP171
|
|
pTagBFP-N
|
FP172
|
|
TagBFP-H2B fusion
|
Human histone H2B is fused to the TagBFP N-terminus. When expressed in mammalian cells, this fusion provides blue fluorescent labeling of histone H2B in living cells.
|
pTagBFP-H2B
|
FP176
|
References:
-
Ai HW, Shaner NC, Cheng Z, Tsien RY, Campbell RE.
Exploration of new chromophore structures leads to the identification of improved blue fluorescent proteins.
Biochemistry. 2007; 46 (20):5904-10. / pmid: 17444659
-
Haas J, Park EC, Seed B.
Codon usage limitation in the expression of HIV-1 envelope glycoprotein.
Curr Biol. 1996; 6 (3):315-24. / pmid: 8805248
-
Subach OM, Gundorov IS, Yoshimura M, Subach FV, Zhang J, Gruenwald D, Souslova EA, Chudakov DM, Verkhusha VV.
Conversion of Red Fluorescent Protein into a Bright Blue Probe.
Chem Biol. 2008; 15 (10):1116-24. doi: 10.1016/j.chembiol.2008.08.006 / pmid: 18940671
|








