|
Red fluorescent protein TagRFP
- Bright red (orange) fluorescence
- Monomeric protein with successful performance in fusions
- Fast maturation, high pH-stability
- Proven suitability to generate stably transfected cell lines
- Recommended for protein labeling, acidic organelle labeling, FRET applications
 |
TagRFP is a monomeric red (orange) fluorescent protein generated from the wild-type RFP from sea anemone Entacmaea quadricolor [Merzlyak et al., 2007]. It possesses bright fluorescence with excitation/emission maxima at 555 and 584 nm, respectively. TagRFP is about three times brighter than mCherry protein [Shaner et al., 2004], which makes it the brightest monomeric red fluorescent protein available so far.
TagRFP is mainly intended for protein labeling and FRET applications. It can also be used for cell and organelle labeling and for tracking the promoter activity.
|
Main properties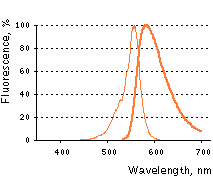
TagRFP normalized excitation (thin line) and emission (thick line) spectra.
Spectra viewer tool
Download TagRFP spectra (xls)
| | CHARACTERISTIC | |
|---|
* Brightness is a product of extinction coefficient and quantum yield, divided by 1000.
** Time to bleach 50% of fluorescent signal brightness.
| | Molecular weight, kDa | 27 | | Polypeptide length, aa | 237 | | Fluorescence color | red (orange) | | Excitation maximum, nm | 555 | | Emission maximum, nm | 584 | | Quantum yield | 0.48 | | Extinction coefficient, M-1cm-1 | 100 000 | | Brightness* | 48.0 | | Brightness, % of EGFP | 148 | | pKa | 3.8 | | Structure | monomer | | Aggregation | no | | Maturation half-time, min | 100 | | Photostability, widefield** | 48 | | Photostability, confocal** | 125 | | Cell toxicity | not observed | | Main advantages | bright red monomeric fluorescent protein | | Possible limitations | medium photostability |
|
|---|
Recommended filter sets and antibodies
TagRFP can be recognized using Anti-tRFP antibody (Cat.# AB233) available from Evrogen.
Recommended Omega Optical filter sets are QMAX-Yellow, XF108-2, XF101-2, and XF111-2. TagRFP can also be detected using TRITC filter set or similar.
Performance and use
TagRFP can be easily expressed and detected in a wide range of organisms. Mammalian cells transiently transfected with TagRFP expression vectors produce bright fluorescence in 10-12 hrs after transfection. No cytotoxic effects or visible protein aggregation are observed. High pH-stability with pKa=3.8 makes it possible to use TagRFP for imaging in acidic organelles, such as late and recycling endosomes and lysosomes.
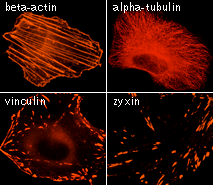
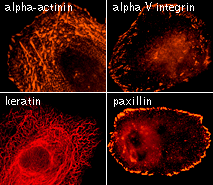
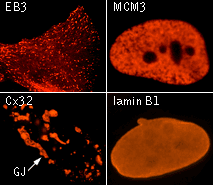
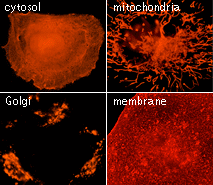 | TagRFP use for cell and organelle labeling.
Microscopic images of HeLa cells transiently transfected with TagRFP and TagRFP-targeted to cellular organelles. Images were kindly provided by Michael W. Davidson (Florida State University).
|
|---|
TagRFP performance in protein fusions has been demonstrated in fibrillarin, vinculin, zyxin, β-actin, α-tubulin, and other models.
TagRFP suitability to generate stably transfected cells has been proven by Marinpharm company.
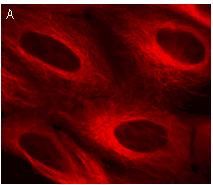 | 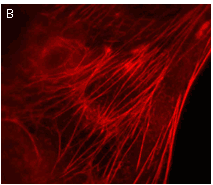 | Stably transfected cell lines expressing TagRFP fusions.
U-205 mammalian cell lines expressing (A) TagRFP fusion with α-tubulin and (B) TagRFP fusion with β-actin. Images were kindly provided by Dr. Christian Petzelt (Marinpharm).
|
|---|
TagRFP can be used in multicolor labeling applications with blue, cyan, green, yellow, and far-red fluorescent dyes.
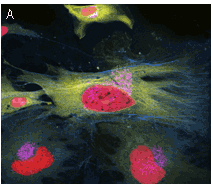 | 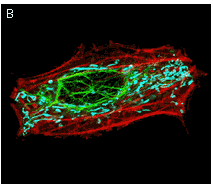 | TagRFP use in multicolor labeling of mammalian cells.
(A) TagCFP-tagged β-actin (cyan), TagYFP-tagged α-tubulin (yellow), TagFP635-H2B fusion (red), and Golgi-targeted TagRFP (violet); (B) mitochondria-targeted TagCFP (cyan), Dendra2-tagged vimentin (green), and TagRFP-tagged β-actin (red). Image (A) was kindly provided by Michael W. Davidson (Florida State University).
|
|---|
TagRFP is ideally suitable for use as an acceptor for FRET with Evrogen green fluorescent protein TagGFP2. The calculated Forster distance (R0 = 5.7 nm) for the TagGFP2-TagRFP pair is one of the largest among the values reported. At the same time, since TagGFP2 and TagRFP emission peaks are spaced by as much as 78 nm, the emission signal for these two proteins can be easily separated in any imaging system. High pH-stability of the both proteins allows using this pair for imaging in acidic organelles.
The excellent performance of TagRFP in FRET application was demonstrated both in vitro and in vivo on the example of FRET-based apoptosis reporter Casper3-GR [Shcherbo et al., 2009].
Available variants and fusions
| Variant | Description | Related vector | Cat.# | Click for image |
|---|
 |
|
Humanized TagRFP
|
TagRFP codon usage is optimized for high expression in mammalian cells [Haas et al., 1996], but it can be successfully expressed in many other heterological systems.
|
pTagRFP-C
|
FP141
|
|
pTagRFP-N
|
FP142
|
|
TagRFP-AS variant
|
TagRFP-AS codon usage is optimized for high expression in Arabidopsis and Saccharomyces. This variant is available in Gateway® entry clones.
|
Gateway® TagRFP-AS-C entry clone
|
FP148
|
|
Gateway® TagRFP-AS-N entry clone
|
FP149
|
|
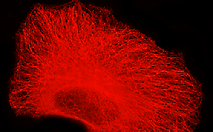
TagRFP-tubulin fusion
|
Human α-tubulin is fused to the TagRFP C-terminus. When expressed in mammalian cells, this fusion provides red (orange) fluorescent labeling of α-tubulin in living cells.
|
pTagRFP-tubulin
|
FP145
|
|
|
TagRFP-mito fusion
|
A mitochondrial targeting sequence (MTS) is fused to the TagRFP N-terminus. MTS was derived from the subunit VIII of human cytochrome C oxidase [Rizzuto et al., 1989; Rizzuto et al., 1995]. When expressed in mammalian cells, this variant provides red (orange) fluorescent labeling of mitochondria.
|
pTagRFP-mito
|
FP147
|

|
|
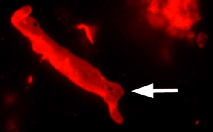
TagRFP-Cx43 fusion
|
Rat connexin 43 is fused to the TagRFP N-terminus. When expressed in mammalian cells, this fusion provides red (orange) fluorescent labeling of connexin 43 in living cells.
|
pTagRFP-Cx43
|
FP364
|
|
|
TagRFP-Golgi fusion
|
Golgi targeting sequence (fragment of human β-1,4-galactosyltransferase) is fused to the TagRFP N-terminus. When expressed in mammalian cells, this variant provides red (orange) fluorescent labeling of Golgi apparatus.
|
pTagRFP-Golgi
|
FP367
|

|
|
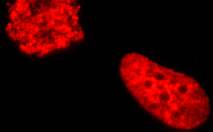
TagRFP-H2B fusion
|
Human histone H2B is fused to the TagRFP N-terminus. When expressed in mammalian cells, this fusion provides red (orange) fluorescent labeling of histone H2B in living cells.
|
pTagRFP-H2B
|
FP368
|
|
References:
-
Haas J, Park EC, Seed B.
Codon usage limitation in the expression of HIV-1 envelope glycoprotein.
Curr Biol. 1996; 6 (3):315-24. / pmid: 8805248
-
Merzlyak EM, Goedhart J, Shcherbo D, Bulina ME, Shcheglov AS, Fradkov AF, Gaintzeva A, Lukyanov KA, Lukyanov S, Gadella TW, Chudakov DM.
Bright monomeric red fluorescent protein with an extended fluorescence lifetime.
Nat Methods. 2007; 4 (7):555-7. / pmid: 17572680
-
Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T.
Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells.
Curr Biol. 1995; 5 (6):635-42. / pmid: 7552174
-
Rizzuto R, Nakase H, Darras B, Francke U, Fabrizi GM, Mengel T, Walsh F, Kadenbach B, DiMauro S, Schon EA.
A gene specifying subunit VIII of human cytochrome c oxidase is localized to chromosome 11 and is expressed in both muscle and non-muscle tissues.
J Biol Chem. 1989; 264 (18):10595-600. / pmid: 2543673
-
Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY.
Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein.
Nat Biotechnol. 2004; 22 (12):1567-72. / pmid: 15558047
-
Shcherbo D, Souslova EA, Goedhart J, Chepurnykh TV, Gaintzeva A, Shemiakina II, Gadella TW, Lukyanov S, Chudakov DM.
Practical and reliable FRET/FLIM pair of fluorescent proteins.
BMC Biotechnol. 2009; 9 :24. doi: 10.1186/1472-6750-9-24 / pmid: 19321010
|