|
Hydrogen peroxide sensor HyPer
- Ratiometric detection of intracellular H2O2 level changes
- High selectivity and sensitivity, no artifactual ROS generation
- Direct expression in cells, easy targeting to various subcellular compartments
- No exogenous chemical compounds required
- Recommended for monitoring H2O2 production inside living cells
 |
Reactive oxygen species (ROS) are tightly involved in normal cell functions as well as in development of a wide variety of pathologies. Commonly used for ROS detection, dichlorofluorescein (DCF) derivatives have several serious disadvantages: they are not specific (i.e. they are sensitive to multiple types of ROS); they cannot be targeted to specific intracellular compartments; and, most importantly, they can produce ROS upon light exposure, which results in artifactual ROS generation and signal amplification.
HyPer is the first fully genetically encoded fluorescent sensor capable of detecting intracellular hydrogen peroxide (H2O2), one of the main ROS generated by cells [Belousov et al., 2006]. Developed on the basis of yellow fluorescent protein inserted into the regulatory domain of E. coli protein OxyR (OxyR-RD) [Choi et al., 2001], HyPer demonstrates submicromolar affinity to hydrogen peroxide and is insensitive to other oxidants tested, such as superoxide, oxidized glutathione, nitric oxide, and peroxinitrite. HyPer does not cause artifactual ROS generation and can be used for detection of fast changes of H2O2 concentration in different cell compartments under various physiological and pathological conditions.
|
Main properties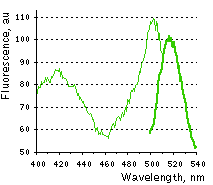
HyPer excitation (thin line) and emission (thick line) spectra.
Download HyPer spectra (xls)
| | CHARACTERISTIC | |
|---|
|
| | Emission maximum, nm | 516 | | Excitation maximum, nm | 420 and 500 | | Fluorescence color | green | | Polypeptide length, aa | 478 | | Molecular weight, kDa | 52 | | Specificity | H2O2 | | Sensitivity | submicromolar H2O2 concentrations | | pKa | 8.5 | | Structure | monomer | | Aggregation | no | | Maturation rate at 37°C | fast |
|
|---|
Recommended filter sets and antibodies
HyPer can be recognized using Anti-Tag(CGY)FP antibody (Cat.# AB121) or Anti-GFP antibody available from Evrogen.
Recommended Omega Optical filter sets for HyPer are QMAX-Green, XF100-2, and XF100-3. It can also be detected using Chroma Technology Corp. filter set 41001 FITC/ RSGFP/ Bodipy/ Fluo 3/ DiO or the similar.
Performance and use
Without H2O2 HyPer has two excitation peaks with maxima at 420 nm and 500 nm, and one emission peak with maximum at 516 nm. Upon exposure to H2O2, the excitation peak at 420 nm decreases proportionally to the increase in the peak at 500 nm, allowing ratiometric measurement of H2O2. Similarly to wild-type OxyR, oxidized HyPer can be reduced inside cells.
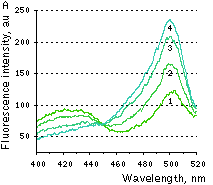 | 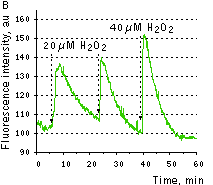 | HyPer response to H2O2 addition.
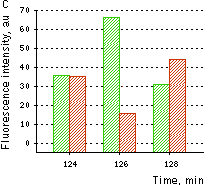
(A) Changes in the excitation spectrum of isolated HyPer in response to hydrogen peroxide addition: Trace 1 — without hydrogen peroxide; trace 2 — 25 nM hydrogen peroxide; trace 3 — 100 nM hydrogen peroxide; trace 4 — 250 nM hydrogen peroxide. Emission was measured at 530 nm. (B) Kinetics of fluorescence (excitation at 490 nm, emission at 530 nm) of HyPer in E. coli cells suspension in the presence of 50 U/ml catalase in response to three successive additions of hydrogen peroxide.
|
|---|
HyPer can be directly expressed by target cells individually or in fusion with a specific localization signal. It successfully folds and remains highly sensitive to hydrogen peroxide both in bacteria and in mammalian cells. If required, stable HyPer transformants can be selected using G418 [Gorman, 1985]. HyPer suitability to generate stably transfected cells has been proven by Marinpharm company.
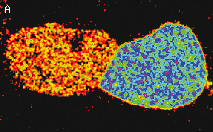
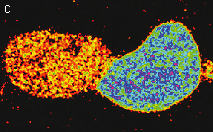
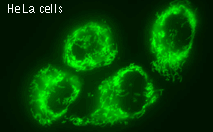 | Stably transfected HeLa cells expressing mitochondria-targeted HyPer.
Image was kindly provided by Dr. Christian Petzelt (Marinpharm).
|
HyPer can be used for monitoring and ratiometric measurement of hydrogen peroxide production in living cells under various physiological and pathological conditions.
Violet and blue excitation light should be applied for monitoring HyPer green emission changes caused by intracellular H2O2 production. Excitation light intensity must be individually determined for a particular biological system and instrumentation used.
Note: Yellow fluorescent core of HyPer undergoes partial photoconversion to a dark state upon irradiation with blue light. It means that an apparent "bleaching" effect occurs at the beginning of time series imaging of cells expressing HyPer protein. Unlike the real bleaching, in the case of HyPer, signal drops to the level of dynamic equilibrium between fluorescent and dark state of the chromophore, and then remains stable.
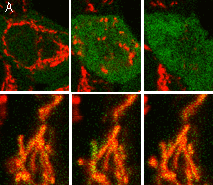
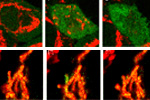
Visualization of hydrogen peroxide production in cytoplasm and mitochondria of HeLa cells during Apo2L/ TRAIL-induced apoptosis: HyPer was targeted to the cytosol of HeLa cells to visualize the hydrogen peroxide production in the cells exposed to the apoptogenic protein Apo2L/TRAIL. The cells were also loaded with tetramethylrhodamine methyl ester (TMRM, 20 nM) to monitor the mitochondrial transmembrane potential.
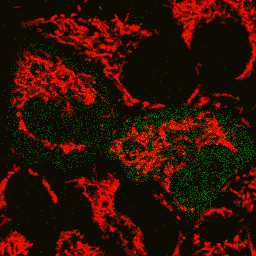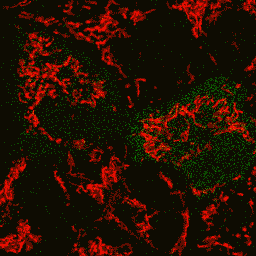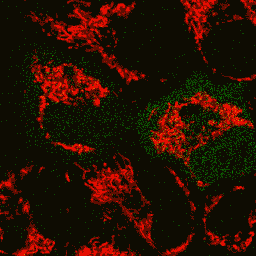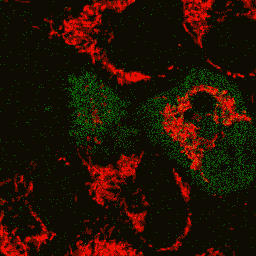 | Image series of cytosolic HyPer (green) and TMRM (red) fluorescence in HeLa cells during apoptosis induced by Apo2L/TRAIL.
HeLa cells expressing the cytosolic form of HyPer were loaded with 20 nM TMRM (to monitor mitochondrial transmembrane potential ΔΨ) and exposed to 400 ng/ml Apo2L/TRAIL. Scanning was performed using 400 Hz line frequency, 512x512 format. Green fluorescent signal was acquired using 488 nm excitation laser line (4% intensity) and detected at 500-520 nm wavelength range. Red fluorescent signal was acquired using 543 nm excitation laser line (12% intensity) and detected at 600-650 nm. Time series speed was 1 frame per 2 min.
|
|---|
Upon stimulation with Apo2L/TRAIL (400 ng/ml), HeLa cells degradation occurred. At 3-5 hrs after Apo2L/TRAIL addition, the cells were observed to change their shape from flat to round with plasma membrane blebs. Using simultaneous visualization of HyPer and TMRM fluorescence, we observed that cytosolic H2O2 started rising in parallel with a loss of the mitochondrial transmembrane potential and a change in the cell shape.
To study changes in the hydrogen peroxide level in mitochondria of HeLa cells treated with Apo2L/TRAIL, mitochondria-targeted HyPer was used. At 1-2 hrs after Apo2L/TRAIL addition, the transmembrane potential of some mitochondria started to oscillate. Simultaneous visualization of HyPer and TMRM fluorescence reveals rising of the H2O2 level during depolarization and decrease of the H2O2 level during repolarization of the mitochondria.
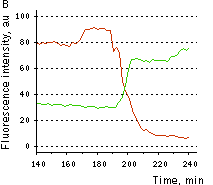
Hydrogen peroxide detection during physiological stimulation: To demonstrate HyPer suitability for detecting low concentrations of H2O2 generated upon physiological stimulation, PC-12 cells which expressed HyPer in the cytoplasm were treated with the nerve growth factor (NGF). H2O2 level in the cytoplasm of stimulated cells was monitored under the same visualization conditions as in the experiment above, but with a higher scanning rate (1 frame per 3 seconds). Two patterns of cellular response were observed for 22 cells in 4 particular experiments. In most cells, H2O2 level started to increase almost immediately after growth factor addition and reached maximum in 3-7 min with the following decrease to the initial level in 10-20 min. Some cells demonstrated biphasic kinetics of hydrogen peroxide production. In such cells, slight initial transient H2O2 level increase was followed by the second higher and rapid increase in H2O2 production; then HyPer fluorescence gradually decreased to the initial level.
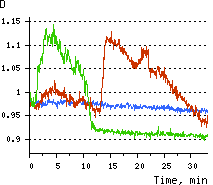 | Dynamics of intracellular production of hydrogen peroxide in PC-12 cells stimulated with 100 ng/ml NGF.
(A-C) Pseudocolored images of cells expressing the cytosolic form of HyPer in 2 min (A), 15 min (B), and 30 min (C) after NGF addition; D — typical timecourses of HyPer fluorescence in cells after NGF stimulation (green and red lines) and in untreated cells (blue line).
|
|---|
Available variants and fusions
| Variant | Description | Related vector | Cat.# | Click for image |
|---|
 |
|
HyPer-AS variant
|
HyPer-AS codon usage is optimized for high expression in Arabidopsis and Saccharomyces. This variant is available in Gateway® entry clone.
|
Gateway® HyPer-AS entry clone*
|
FP943
|
|
HyPer-Cyto
|
HyPer comprises fluorescent part which codon usage is optimized for high expression in mammalian cells [Haas et al., 1996] and OxyR part from E. coli with autogenic codon usage. Effective expression of HyPer occures in both bacteria and mammalian cells.
When expressed in mammalian cells, this variant is evenly distributed throughout the cytosol and the nucleus.
|
pHyPer-cyto*
|
FP941
|
|
HyPer-dMito
|
Two copies of mitochondrial targeting sequence derived from subunit VIII precursor of human cytochrome C oxidase [Rizzuto et al., 1989; Rizzuto et al., 1995] are fused to HyPer N-terminus. When expressed in mammalian cells, this variant is localized in mitochondria.
|
pHyPer-dMito*
|
FP942
|
|
HyPer-nuc
|
Three copies of the nuclear localization signal [Fischer-Fantuzzi and Vesco, 1988] are fused to the HyPer C-terminus. When expressed in mammalian cells, this variant is localized in the nuclei.
|
pHyPer-nuc*
|
FP944
|
|
References:
-
Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S.
Genetically encoded fluorescent indicator for intracellular hydrogen peroxide.
Nat Methods. 2006; 3 (4):281-6. / pmid: 16554833
-
Choi H, Kim S, Mukhopadhyay P, Cho S, Woo J, Storz G, Ryu S.
Structural basis of the redox switch in the OxyR transcription factor.
Cell. 2001; 105 (1):103-13. / pmid: 11301006
-
Fischer-Fantuzzi L, Vesco C.
Cell-dependent efficiency of reiterated nuclear signals in a mutant simian virus 40 oncoprotein targeted to the nucleus.
Mol Cell Biol. 1988; 8 (12):5495-503. / pmid: 2854199
-
Gorman C.
High efficiency gene transfer into mammalian cells.
In DNA cloning: A Practical Approach, Vol. II. Ed. D. M. Glover. (IRL Press, Oxford, U.K.). 1985; 143-90.
-
Haas J, Park EC, Seed B.
Codon usage limitation in the expression of HIV-1 envelope glycoprotein.
Curr Biol. 1996; 6 (3):315-24. / pmid: 8805248
-
Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T.
Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells.
Curr Biol. 1995; 5 (6):635-42. / pmid: 7552174
-
Rizzuto R, Nakase H, Darras B, Francke U, Fabrizi GM, Mengel T, Walsh F, Kadenbach B, DiMauro S, Schon EA.
A gene specifying subunit VIII of human cytochrome c oxidase is localized to chromosome 11 and is expressed in both muscle and non-muscle tissues.
J Biol Chem. 1989; 264 (18):10595-600. / pmid: 2543673
|