
|
||||||||||
|
||||||||||

|
Gateway® |
||||||||||||||||||||||||||||||||||||||||||||||||||||
The vector sequence has been compiled using the information from sequence databases, published literature, and other sources, together with partial sequences obtained by Evrogen. This vector has not been completely sequenced. |
|||||||||||||||||||||||||||||||||||||||||||||||||
 Download
|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Vector description
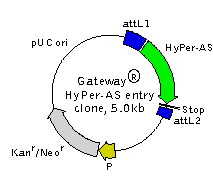
Gateway® HyPer-AS entry clone is a vector containing HyPer gene variant with codon usage optimized for high expression in Arabidopsis and Saccharomyces (see reporter description). HyPer coding sequence is flanked by attL1 and attL2 sites allowing easy site-specific recombination. The Invitrogen Gateway® Technology provides a rapid and highly efficient way to transfer the HyPer gene into a number of Gateway® destination vectors for expression in different experimental systems.
To increase mRNA translation efficiency, Kozak consensus translation initiation site is generated upstream of the HyPer coding sequence [Kozak, 1987].
The vector backbone contains pUC origin of replication and kanamycin resistance gene (Kanr) for propagation and selection in
LR site-specific recombination
Please refer to Invitrogen Gateway® Technology description for detailed instructions regarding LR site-specific recombination reaction. In general, to transfer HyPer gene into the destination vector you will need:
- Purified plasmid DNA of Gateway® HyPer-AS
- A destination vector of choice
- Invitrogen LR ClonaseTM II enzyme mix (Invitrogen Cat.# 11791-020)
- Proteinase K solution (supplied with the LR ClonaseTM II enzyme mix)
- TE-Buffer, pH 8.0 (10 mM Tris-HCl, pH 8.0, 1 mM EDTA)
- Appropriate chemically competent
- Appropriate selective plates.
Propagation in
Suitable host strains for propagation in
Location of features
attL1 site: 14-113
Kozak translation initiation site: 129-139
HyPer-AS: 136-1572
attL2 site: 1598-1697
Kanamycin resistance gene: 2922-3716
pUC origin of replication: 4301-4944
References:
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987; 15 (20):8125-48. / pmid: 3313277
Notice to Purchaser:
HyPer-related materials (also referred to as "Products") are intended for research use only. Some elements of these materials may be covered by third party patents issued and applicable in certain countries. No license under these patents is conveyed expressly or by implication to the recipient of the materials. Users of these materials may be required to obtain a patent license depending upon the particular application and country in which the materials are received or used.
Invitrogen Gateway® Technology: please see Limited Use Label License No. 19: Gateway® Cloning Products.
|
Copyright 2002-2023 Evrogen. All rights reserved. Evrogen JSC, 16/10 Miklukho-Maklaya str., Moscow, Russia, Tel +7(495)988-4084, Fax +7(495)988-4085, e-mail:evrogen@evrogen.com |


