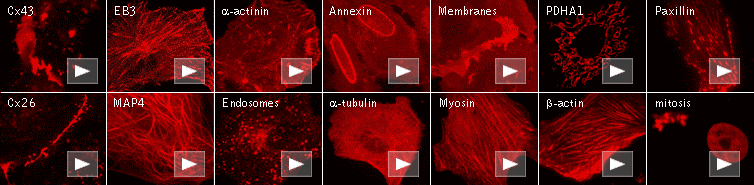
|
Red fluorescent protein FusionRed
- Superior performance in fusions
- Low cytotoxicity
- Fast maturation, high pH-stability and photostability
- Proven suitability to generate stably transfected cell lines
- Recommended for protein labeling and long-term experiments
Performance and use
FusionRed can be easily expressed and detected in a wide range of organisms. Mammalian cells transiently transfected
with FusionRed expression vectors produce bright fluorescence in 10-12 hours after transfection. FusionRed
performance in fusions has been demonstrated in a number of models.
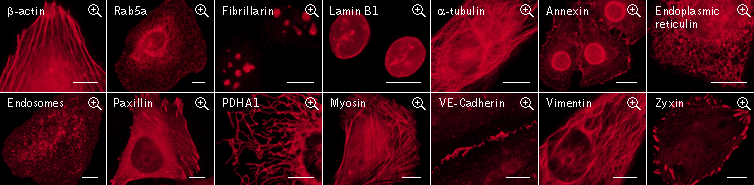
|
FusionRed use for protein labeling in mammalian cells.
Scale bars represent 10 μm. Data from Shemiakina et al., 2012
|
Labeling efficiency testing
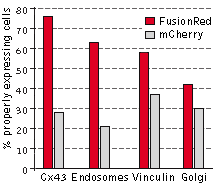 |
The performance of FusionRed and mCherry was directly compared in HeLa CCL2 cells for the following
targets: connexin-43, endosomes, vinculin, and the Golgi complex. In this experiment, the cells were
transfected with each of the four target fusions, fixed, and then compared for localization efficiency,
which was determined by calculating the percentage of properly expressing cells versus the total number
of transfected cells. FusionRed demonstrated clear advantage in all four fusions. Data from Shemiakina
et al., 2012
|
Cytotoxicity testing in HeLa cells
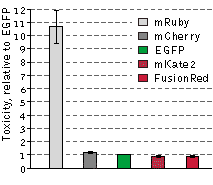 |
The cytotoxicity of FusionRed relative to selected red fluorescent proteins and EGFP was evaluated in
the following experiment: HeLa cells were transfected with appropriate vectors encoding EGFP or one of
the following red fluorescent proteins: mRuby, FusionRed, mKate2 or mCherry. Next, the EGFP-expressing
cells were mixed with those expressing one of the RFPs, resulting in 4 separate cell mixtures: EGFP and
mRuby, EGFP and FusionRed, EGFP and mKate2, and EGFP and mCherry. 48 hours after transfection, the
green-to-red cell ratios were calculated utilizing flow cytometry and each of the cell mixtures were
then plated into 3 plates. After additional 92 hour incubation, the green-to-red cell ratios were
recalculated. Because only living cells were counted for this experiment, the difference between the
ratios before and after the incubation can be assumed to accurately reflect RFP toxicity versus EGFP.
mRuby exhibited a more than 10-fold higher cytotoxicity level compared to EGFP, while the remaining RFPs
were almost as cytotoxic as EGFP in this experiment. Data from Shemiakina et al., 2012
|
Cytotoxicity testing in Xenopus laevis tadpoles
The eye formation is a complex multistage process
that depends on many mechanisms, any disturbance of which may result in an abnormal phenotype. Here eye development
was used as a sensitive readout of possible abnormal course of embryogenesis caused by expression of EGFP,
FusionRed, and mCherry fusions. In these experiments, actin and vimentin fusions of EGFP and FusionRed were only
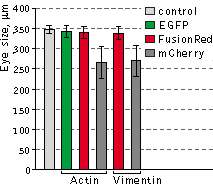
slightly more toxic than the uninjected controls, while expression of mCherry fusions led to notable decrease of the
average eye size. Moreover, in many cases (80% of tadpoles with the reduced eye size), the reduced eye phenotype was
accompanied by varying degree of an unclosed optic fissure, indicating an abnormal eye development.
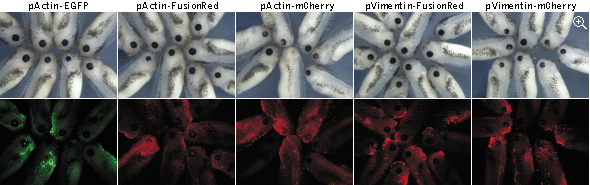
|
Stage 41 Xenopus laevis tadpoles expressing fluorescent protein fusions. Top panels: white light;
bottom panels: fluorescence. Data from Shemiakina et al., 2012
|
 |
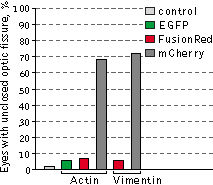 |
Left graph: Mean values of eye size of Xenopus laevis embryos injected with plasmids expressing
different fluorescent protein fusions.
Right graph: Percentage of reduced eyes with unclosed optic fissure.
Data from Shemiakina et al., 2012
|
Long-term expression
The excellent suitability of FusionRed for long-term experiments was proved both in
experiments with stably transfected cell lines and in transgenic animals. In addition to its low cytotoxicity,
FusionRed does not show abnormal lysosomal localization typical for many fluorescent proteins in long-term
expression.
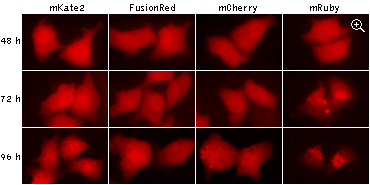
|
Being expressed in HeLa cells, both mKate2 and FusionRed remain evenly localized in cytoplasm after 96
hours of cultivation, while mCherry and mRuby show abnormal lysosomal localization. Data from Shemiakina
et al., 2012
|
References:
-
Shemiakina II, Ermakova GV, Cranfill PJ, Baird MA, Evans RA, Souslova EA, Staroverov DB, Gorokhovatsky AY, Putintseva EV, Gorodnicheva TV, Chepurnykh TV, Strukova L, Lukyanov S, Zaraisky AG, Davidson MW, Chudakov DM, Shcherbo D.
A monomeric red fluorescent protein with low cytotoxicity.
Nat Commun. 2012; 3 :1204. doi: 10.1038/ncomms2208 / pmid: 23149748
|