
|
Caspase-3 apoptosis sensor Casper3-BG
- Early detection of Caspase-3 activity onset
- High sensitivity
- Direct expression in cells
- No exogenous chemical compounds required
- Recommended for early detection of apoptosis
 |
Caspase-3 (CPP32, apopain, YAMA), a member of asparate-specific cysteinyl proteases (or caspases) family, is a key mediator of apoptosis of mammalian cells [Kothakota et al., 1997]. Caspase-3 is activated during the early stages of apoptosis by self-proteolysis and/or cleavage by another protease. Active caspase-3 cleaves and activates caspases and many other cellular proteins, leading to apoptotic chromatin condensation and DNA fragmentation in all cell types examined [Porter and Janicke, 1999].
Casper3-BG is a FRET based sensor that can be used for detection of caspase-3 mediated apoptosis in living cells. The sensor consists of blue and green fluorescent proteins, TagBFP and TagGFP2, connected by the linker containing caspase-3 cleavage sequence, DEVD. Good overlap between the emission spectrum of TagBFP and the absorbance spectra of TagGFP2 ensures efficient FRET between these proteins [Subach et al., 2008].
The activation of caspase-3 during apoptosis leads to cleavage of DEVD sequence and elimination of FRET that can be detected as a decrease in green emission of TagGFP2 and a simultaneous increase in blue emission of TagBFP.
|
Main properties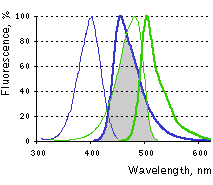
Excitation (thin lines) and emission (thick lines) spectra of TagBFP (blue) and TagGFP2 (green) are shown individually. Spectral overlap is filled with gray.
Download Casper3-BG spectra (xls)
| | CHARACTERISTIC | |
|---|
|
| | FRET donor | TagBFP | | Fluorescence color | blue | | Excitation maximum, nm | 402 | | Emission maximum, nm | 457 | | Brightness, % of EGFP | 99 | | pKa | 2.7 | | FRET acceptor | TagGFP2 | | Fluorescence color | green | | Excitation maximum, nm | 483 | | Emission maximum, nm | 506 | | Brightness, % of EGFP | 105 | | pKa | 5.0 | | Calculated Förster distance R0 | 5.25 | | FRET efficiency E | 0.57 | | Specificity | caspase-3 activity | | Response | elimination of FRET | | Polypeptide length, aa | 490 | | Molecular weight, kDa | 55 |
|
|---|
Recommended filter sets
The set of filters from Chroma Technology Corp. (403/12 nm exciter, part #74673, 457/50 nm emitter, part #66974, and dichroic mirror, part #86100) or similar.
Performance and use
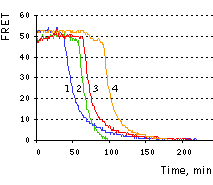
The excellent performance of Casper3-BG sensor has been demonstrated in vivo on the example of HeLa cells staurosporine-induced apoptosis [Subach et al., 2008]. The two-filter method of sensitized FRET measurements [Gordon et al., 1998] on a pixel-by-pixel basis was applied, as described in [Galperin et al., 2004]. The initial mean FRET efficiency in vivo normalized to donor fluorescence was 51.5%. Following 40-80 min exposure to 1 mM staurosporine, the FRET gradually dropped to zero before the shrinking of cells characteristic to apoptosis. The large FRET efficiency of the TagBFP-TagGFP2 pair enabled the detection of even weak proteolitic activity in each cell at the beginning of apoptosis, when only a fraction of the substrate was cleaved.
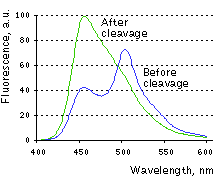 | Change in Casper3-BG emission spectra upon the cleavage of DEVD sequence in vitro.
|
|---|
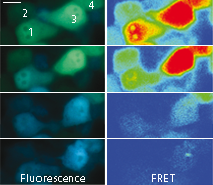 |  | Imaging of FRET intensity in staurosporine-treated HeLa cells.
Cells treated with staurosporine are shown as overlaid fluorescent images of blue and green channels (left panels). The corrected FRET signals are shown in pseudocolor (right panels). Scale bar represents 10 μm.
On the right, time course of corrected FRET signal for the four cells is shown.
|
|---|
References:
-
Galperin E, Verkhusha VV, Sorkin A.
Three-chromophore FRET microscopy to analyze multiprotein interactions in living cells.
Nat Methods. 2004; 1 (3):209-17. / pmid: 15782196
-
Gordon GW, Berry G, Liang XH, Levine B, Herman B.
Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy.
Biophys J. 1998; 74 (5):2702-13. / pmid: 9591694
-
Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT.
Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis.
Science. 1997; 278 (5336):294-8. / pmid: 9323209
-
Porter AG, Janicke RU.
Emerging roles of caspase-3 in apoptosis.
Cell Death Differ. 1999; 6 (2):99-104. / pmid: 10200555
-
Subach OM, Gundorov IS, Yoshimura M, Subach FV, Zhang J, Gruenwald D, Souslova EA, Chudakov DM, Verkhusha VV.
Conversion of Red Fluorescent Protein into a Bright Blue Probe.
Chem Biol. 2008; 15 (10):1116-24. doi: 10.1016/j.chembiol.2008.08.006 / pmid: 18940671
|









