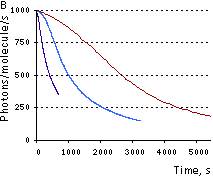
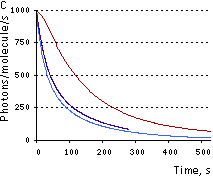
|
||||||||||
|
||||||||||

mKate2
SUPPORTRESOURCES |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
mKate2 is the next generation of far-red fluorescent protein TagFP635 (mKate) [Shcherbo et al., 2007; Shcherbo et al., 2009]. Possessing fluorescence with excitation/emission maxima at 588 and 633 nm, mKate2 is almost 3-fold brighter than TagFP635 and is 10-fold brighter than mPlum at physiological pH 7.5. Within the optical window optimal for light penetration in living tissues, calculated brightness of mKate2 is at least 2-fold higher compared to any monomeric fluorescent protein reported to date. mKate2 is characterized by complete and fast chromophore maturation at 37°C with maturation half-time <20 min (versus 40 min for mCherry). It is more photostable under both widefield and confocal illumination than other monomeric far-red proteins, including TagFP635, mRaspberry and mPlum. The high brightness, far-red emission spectrum, excellent pH resistance and photostability, coupled with low toxicity demonstrated in transgenic Xenopus laevis embryos, make mKate2 a superior fluorescent tag for imaging in living tissues. mKate2 is mainly intended for protein labeling. Its far-red fluorescence allows easy and reliable separation from standard green fluorescent labels in dual-color high-throughput assays. |
Main properties
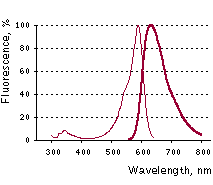
mKate2 normalized excitation (thin line) and emission (thick line) spectra. |
| ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
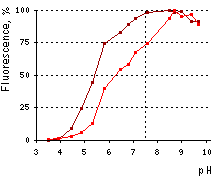 | pH stability of mKate2 (brown line) and TagFP635 (red line) fluorescence.
|
|---|
Recommended filter sets and antibodies
mKate2 can be recognized using Anti-tRFP antibody (Cat.# AB233) available from Evrogen.
Recommended Omega Optical filter sets are QMAX-Red and XF102-2. mKate2 can also be detected using Texas Red filter sets or similar.
Performance and use
mKate2 can be easily expressed and detected in a wide range of organisms. Mammalian cells transiently transfected with mKate2 expression vectors produce bright fluorescence in 10-12 hours after transfection. No cytotoxic effects or visible protein aggregation are observed.
mKate2 performance in fusions has been demonstrated in β-actin and other models.
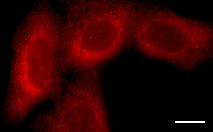
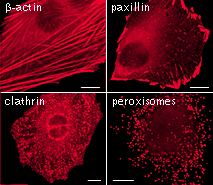 |  | mKate2 use for protein labeling in mammalian cells.Scale bar represents 10 μm. Images from Shcherbo et al., 2009. |
|---|
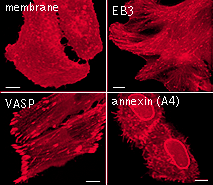
 |  | Live cell imaging of protein dynamics with mKate2.
|
|---|
mKate2 is the superior fluorescent monomeric tag for imaging in living tissues. It has emission maximum at 635 nm optimal for deep imaging of animal tissues, and is more bright, photostable and pH-stable than other cloned far-red monomeric fluorescent proteins.
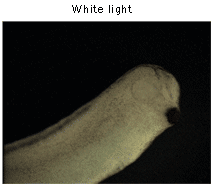
To verify the low toxicity of mKate2 in transgenic animals, mKate2 was expressed under the control of Xanf1 promoter in Xenopus laevis embryos. As expected, bright red fluorescence in the forehead region, including eyes, the forebrain and nasal placodes was observed.
 |  |  | |
|---|---|---|---|
Imaging of mKate2 in Xenopus laevis embryos.Expression of mKate2 under the control of Xanf1 promoter in the transgenic embryos at stage 28 is specifically localized in the forehead region, including eyes, the forebrain and nasal placodes. The embryo is shown from the right side, dorsal to the top and left. Data courtesy of Dr. A. Zaraisky, Institute of Bioorganic Chemistry, RAS (Moscow, Russia). | |||
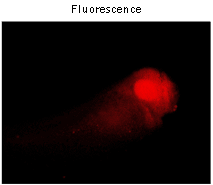
To compare brightness and maturation rate of TagFP635 and mKate2 in vivo, Xenopus laevis embryos were microinjected with pTagFP635-N and pmKate2-N vectors at the stage of two blastomeres. Living embryos were photographed from the animal pole side at the middle gastrula stages (10.5 hours after fertilization). As expected, embryos microinjected with pmKate2-N demonstrated superior brightness.
 | Comparison of TagFP635 and mKate2 in developing Xenopus laevis embryos.Data courtesy of Dr. A. Zaraisky, Institute of Bioorganic Chemistry, RAS (Moscow, Russia). |
|---|
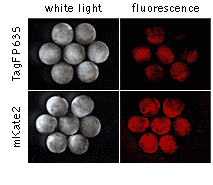
In addition, to test in an embryonic model the performance of mKate2 in a targeting protein fusion, we generated transgenic Xenopus laevis embryos bearing a CMV-mKate2-zyxin fusion construct. Despite quite extensive and ubiquitous expression of mKate2-zyxin under the control of the CMV promoter, these embryos appear normal and healthy indicating that mKate2 exerts a low toxic effect on living cells in transgenic organisms.
 | 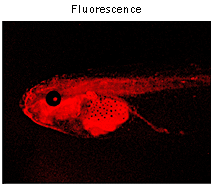 | Expression of mKate2-zyxin in Xenopus laevis embryo.Data courtesy of Dr. A. Zaraisky, Institute of Bioorganic Chemistry, RAS (Moscow, Russia). |
|---|
mKate2 can be used in multicolor labeling applications with blue, cyan, green, yellow, and red (orange) fluorescent dyes. High pH-stability with pKa=5.4 makes it possible to use mKate2 for imaging in acidic organelles, such as late and recycling endosomes and lysosomes.
| Variant | Description | Related vector | Cat.# | Click for image |
|---|---|---|---|---|
 |
||||
| Humanized mKate2 | mKate2 codon usage is optimized for high expression in mammalian cells [Haas et al., 1996], but it can be successfully expressed in many other heterological systems. |
|
FP181 | |
|
|
FP182 | |||
| mKate2-actin fusion |
Human
|
|
FP184 |

|
| mKate2-f-mem fusion | 20 amino acid farnesylation signal from c-Ha-Ras is fused to the mKate2 C-terminus. When expressed in mammalian cells, this fusion provides far-red fluorescent labeling of plasma membrane. |
|
FP186 |
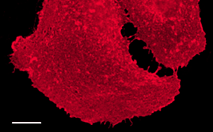
|
| mKate2-mito fusion | A mitochondrial targeting sequence (MTS) is fused to the mKate2 N-terminus. MTS was derived from the subunit VIII of human cytochrome C oxidase [Rizzuto et al., 1989; Rizzuto et al., 1995]. When expressed in mammalian cells, this variant provides far-red fluorescent labeling of mitochondria. |
|
FP187 |
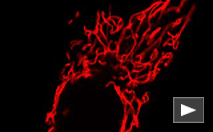
|
| mKate2-H2B fusion | Human histone H2B is fused to the mKate2 N-terminus. When expressed in mammalian cells, this fusion provides far-red fluorescent labeling of histone H2B in living cells. |
|
FP311 |
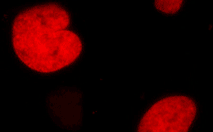
|
| mKate2-lyso fusion | Rat Lysosomal Associated Membrane Protein 1 (LAMP-1) is fused to the mKate2 N-terminus. When expressed in mammalian cells, this fusion provides far-red fluorescent labeling of lysosomes. |
|
FP312 |
|
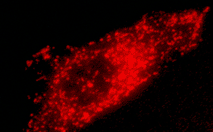
| mKate2-peroxi fusion | Peroxisomal targeting signal [Gould et al., 1989] encoding tripeptide SKL is fused to the 3' end of mKate2 sequence. This tripeptide targets the fusion protein to the matrix of peroxisomes. |
|
FP313 |
|
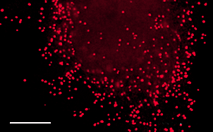
| mKate2-endo fusion | Human RhoB GTPase is fused to the mKate2 C-terminus. When expressed in mammalian cells, this fusion provides far-red fluorescent labeling of vesicles of the endocytic pathway. |
|
FP314 |
|
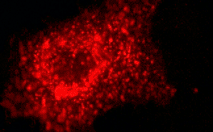
| mKate2-clathrin fusion | Human clathrin LCB is fused to the mKate2 C-terminus. When expressed in mammalian cells, this fusion provides far-red fluorescent labeling of clathrin LCB in living cells. |
|
FP322 |
|
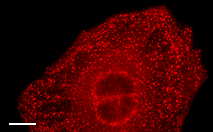
| mKate2-ER fusion | Signal sequence of calreticulin is fused to the mKate2 N-terminus and the endoplasmic reticulum retention sequence KDEL is fused to the mKate2 C-terminus. When expressed in mammalian cells, this fusion provides far-red fluorescent labeling of the lumen of the endoplasmic reticulum. |
|
FP324 |
|
References:
- Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989; 264 (36):21522-8. / pmid: 2600080
- Gould SJ, Keller GA, Hosken N, Wilkinson J, Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989; 108 (5):1657-64. / pmid: 2654139
- Haas J, Park EC, Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996; 6 (3):315-24. / pmid: 8805248
- Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987; 48 (5):899-907. / pmid: 3545499
- Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T. Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells. Curr Biol. 1995; 5 (6):635-42. / pmid: 7552174
- Rizzuto R, Nakase H, Darras B, Francke U, Fabrizi GM, Mengel T, Walsh F, Kadenbach B, DiMauro S, Schon EA. A gene specifying subunit VIII of human cytochrome c oxidase is localized to chromosome 11 and is expressed in both muscle and non-muscle tissues. J Biol Chem. 1989; 264 (18):10595-600. / pmid: 2543673
- Shcherbo D, Merzlyak EM, Chepurnykh TV, Fradkov AF, Ermakova GV, Solovieva EA, Lukyanov KA, Bogdanova EA, Zaraisky AG, Lukyanov S, Chudakov DM. Bright far-red fluorescent protein for whole-body imaging. Nat Methods. 2007; 4 (9):741-6. / pmid: 17721542
- Shcherbo D, Murphy CS, Ermakova GV, Solovieva EA, Chepurnykh TV, Shcheglov AS, Verkhusha VV, Pletnev VZ, Hazelwood KL, Roche PM, Lukyanov S, Zaraisky AG, Davidson MW, Chudakov DM. Far-red fluorescent tags for protein imaging in living tissues. Biochem J. 2009; 418 (3):567-74. doi: 10.1042/BJ20081949 / pmid: 19143658
|
Copyright 2002-2023 Evrogen. All rights reserved. Evrogen JSC, 16/10 Miklukho-Maklaya str., Moscow, Russia, Tel +7(495)988-4084, Fax +7(495)988-4085, e-mail:evrogen@evrogen.com |