
|
||||||||||
|
||||||||||

NirFP
SUPPORTRESOURCES |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
NirFP (scientific name eqFP670) is a red-shifted variant of TurboFP635 (Katushka) [Shcherbo et al., 2010]. NirFP is characterized by a strong bathochromic shift, with excitation and emission peaks at 605 nm and 670 nm, respectively. It is currently the most red-shifted fluorescent protein available, with approximately half of emission falling in the infrared part of the spectrum. The brightness of NirFP in the 700 – 900 nm region upon excitation at 635 nm is about 4 times higher than the brightness of TurboFP635 and 1.6 times higher than the brightness of TurboFP650. The protein does not show residual short wavelength fluorescence of intermediate or alternative chromophore forms, in contrast to E2-Crimson [Strack et al., 2009], which exhibits a second bright blue emission peak, and mNeptune [Lin et al., 2009], which has a pronounced green peak. NirFP is characterized by high pH stability and extremely high photostability that should allow for accumulation of the fluorescent signal over long exposure times. NirFP is recommended for multicolor applications. It can also be used for whole body imaging utilizing long wavelengths for excitation (e.g., 633 or 635 nm laser lines). |
Main properties
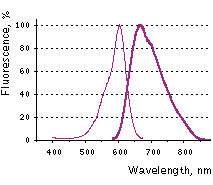
NirFP normalized excitation (thin line) and emission (thick line) spectra. |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Recommended filter sets and antibodies
NirFP can be recognized using Anti-tRFP antibody (Cat.# AB233) available from Evrogen.
The optimal excitation/emission ranges for NirFP visualization are:
excitation: 560-620 nm
emission: 630-850 nm
Therefore, many common filter sets used for visualization of red and far-red fluorescent proteins, Texas Red, Allophycocyanin and Cy5 (wide excitation), can be used with NirFP as well.
The recommended filter sets for gathering the maximal signal from NirFP alone:
Chroma Technology Corp.: 11010v2 Yellow, 41024 Cy5 Longpass Emission
Semrock : LF594/LP-A (especially with 594 nm laser excitation).
Omega Optical: XF102-2, XF40-2
The recommended filter sets for spectral separation with orange-red fluorescent proteins*, such as TurboRFP or TagRFP:
Chroma Technology Corp.: 41024 Cy5 Longpass Emission, 49006 ET - Cy5
Semrock: Cy5-4040A, Cy5-4040B, LF594/LP-A
Omega Optical: XF110-2
* The final choice of the filter set should be made basing on the spectral characteristics of the second fluorescent protein.
Performance and use
NirFP can be easily visualized within living tissues. Mammalian cells transiently transfected with NirFP expression vectors produce fluorescence in 48 hrs after transfection. No cytotoxic effects or visible protein aggregation are observed.
Despite its dimeric structure, NirFP can be used in some fusions. However, for protein labeling applications we recommend using specially optimized monomeric TagFPs.
NirFP can be used in multicolor labeling applications with blue, cyan, green, yellow, and red (orange) fluorescent dyes.
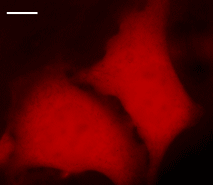 | HeLa cells transiently transfected with pNirFP-N vector.Widefield Leica AFLX 6000 microscope, 63x objective, after 3 days of incubation. Scale bar, 10 μm. Image from Shcherbo et al., 2010. |
|---|
| Variant | Description | Related vector | Cat.# | |
|---|---|---|---|---|
 | ||||
| Humanized NirFP | NirFP codon usage is optimized for high expression in mammalian cells [Haas et al., 1996], but it can be successfully expressed in many other heterological systems. Evrogen mammalian expression vectors comprising multiple cloning sites at the 5�- or 3�-end of NirFP coding sequence allow easy generation of fusions of interest. |
|
FP743 | |
References:
- Haas J, Park EC, Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996; 6 (3):315-24. / pmid: 8805248
- Lin MZ, McKeown MR, Ng HL, Aguilera TA, Shaner NC, Campbell RE, Adams SR, Gross LA, Ma W, Alber T, Tsien RY. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem Biol. 2009; 16 (11):1169-79. doi: 10.1016/j.chembiol.2009.10.009 / pmid: 19942140
- Shcherbo D, Shemiakina II, Ryabova AV, Luker KE, Schmidt BT, Souslova EA, Gorodnicheva TV, Strukova L, Shidlovskiy KM, Britanova OV, Zaraisky AG, Lukyanov KA, Loschenov VB, Luker GD, Chudakov DM. Near-infrared fluorescent proteins. Nat Methods. 2010; 7 (10):827-9. doi: 10.1038/nmeth.1501 / pmid: 20818379
- Strack RL, Hein B, Bhattacharyya D, Hell SW, Keenan RJ, Glick BS. A rapidly maturing far-red derivative of DsRed-Express2 for whole-cell labeling. Biochemistry. 2009; 48 (35):8279-81. doi: 10.1021/bi900870u / pmid: 19658435
|
Copyright 2002-2023 Evrogen. All rights reserved. Evrogen JSC, 16/10 Miklukho-Maklaya str., Moscow, Russia, Tel +7(495)988-4084, Fax +7(495)988-4085, e-mail:evrogen@evrogen.com |



