
|
Calcium ion sensor Case12
- High dynamic range detection of intracellular Ca2+ level changes
- High selectivity and sensitivity, relatively high pH stability
- Fast maturation, high brightness of fluorescent response
- Direct expression in cells, easy targeting to various subcellular compartments
- No exogenous chemical compounds required
- Recommended for monitoring changes in Ca2+ concentration inside living cells
 |
Case12 is a high dynamic range genetically encoded fluorescent sensor for direct measurement of changes of intracellular Ca2+ under various physiological and pathological conditions [Souslova et al., 2007]. The sensor is sensitive to changes of calcium concentration in a physiological range from a hundred nanomoles to micromoles with a high signal-to-noise ratio. Binding of Ca2+ is fast and reversible, allowing monitoring of high-frequency Ca2+ oscillations. In response to Ca2+ concentration rise, Case12 shows up to 12-fold increase of fluorescence brightness. Fluorescence of Case12 is characterized by single excitation/emission maxima peaked at 491/516 nm.
Case12 is recommended for monitoring change of calcium concentration inside living cells during various physiological and pathological conditions.
|
Main properties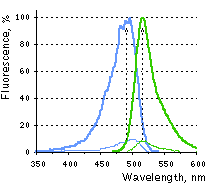
Case12 normalized excitation (blue) and emission (green) spectra without Ca2+ (thin line) and in the presence of 1 mM of Ca2+ (thick line).
Download Case12 spectra (xls)
| | CHARACTERISTIC | |
|---|
|
| | Emission maximum, nm | 516 | | Excitation maximum, nm | 491 | | Fluorescence color | green | | Polypeptide length, aa | 415 | | Molecular weight, kDa | 46.4 | | Specificity | Ca2+ | | Kd for Ca2+ | 1 μM | | pKa | 7.2 | | Structure | monomer | | Aggregation | no | | Maturation rate at 37°C | fast |
|
|---|
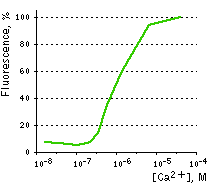 | Ca2+ titration curves.
The apparent Kd for calcium ions binding was found to be 1 μM, which lies within the physiological range of calcium ions concentrations. Image from Souslova et al., 2007.
|
|---|
Recommended filter sets and antibodies
Case12 can be recognized using Anti-Tag(CGY)FP antibody (Cat.# AB121) or Anti-GFP antibody available from Evrogen.
We recommend standard GFP filter sets. Appropriate Omega Optical filter sets for Case12 are QMAX-Green, XF100-2 and XF100-3. It can also be detected using Chroma Technology Corp. filter sets 41001, 41017, 41020, 41025 or similar.
Performance and use
The common weak point of conventional calcium sensors is their low pH stability. For example, pKa (meaning of pH at which fluorescence brightness is 50% of maximum) for Pericams reaches as high as 8.0. Therefore, at physiological pH (7.2-7.5) such sensors exhibit low brightness and dynamic range [Nagai et al., 2001]. In contrast, the pKa of Case12 is 7.2 (in the presence of 10 μM Ca2+) close to that reported for G-CaMP [Nakai et al., 2001]. This relatively high pH stability makes Case12 well suitable for in vivo use.
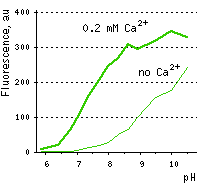 | Dependence of Case12 fluorescence on pH in the presence (thick line) and in the absence (thin line) of Ca2+.
Image from Souslova et al., 2007.
|
|---|
Case12 is characterized by fast maturation at 37°C and bright fluorescent response to Ca2+. It can be directly expressed by target cells, both individually and in fusion with a specific localization signal. No aggregation is observed upon long-term (5 days) expression of Case12 in transiently transfected cells.
Monitoring changes in green emission of Case12 in response to intracellular changes of Ca2+ concentration should be carried out by excitation by blue light (488 nm laser line or standard GFP filter set). Emission can be collected at approximately 500-540 nm. Intensity of excitation light should be individually determined for particular biological system and instrumentation. In general, we recommend that you minimize excitation light intensity and duration.
Note: Yellow fluorescent core of Case12 undergoes partial photoconversion to a dark state upon irradiation with blue light. It means that an apparent "bleaching" effect occurs at the beginning of time series imaging of cells expressing Case12 protein. Unlike the real bleaching, in the case of Case12, signal drops to the level of dynamic equilibrium between fluorescent and dark state of the chromophore, and then remains stable.
Maximum dynamic range in HeLa cells: HeLa cells transfected with Case12 showed relatively weak green fluorescence, which was detected with a Leica microscope DM IRE2, confocal TCS-SP2, objective HCX-PL-APO-63x/1.40-0.60/OIL. Addition of 20 μM calcium ionophore A23187, allowing calcium to enter cells (2 mM Ca2+ in the medium), resultes in 5-6-fold increase in green fluorescence brightness. Subsequent addition of 20 mM EGTA removes Ca2+ and decreases the fluorescence signal close to baseline level, with the final contrast of 11-12-fold.
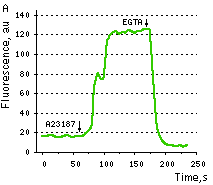 | 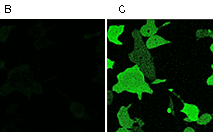 | Testing Case12 in living cells.
(A) Typical response of HeLa cells expressing Case12 to calcium ionophore A23187. (B, C) HeLa cells expressing Case12 shown before (B) and after (C) ionophore addition. Image from Souslova et al., 2007.
|
|---|
Monitoring of Ca2+ changes under physiological conditions: Mammalian cells expressing Case12 display a nice high dynamic range response upon addition of ATP at a final concentration of 100 μM. This experiment clearly showes that Case12 fluorescence response to Ca2+ oscillations is fast and reversible. It also demonstrates that the sensor responds to changes in Ca2+ concentration in living cells in the nanomolar range.
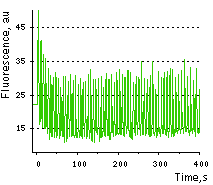 | Fluorescence changes of human melanoma-derived M21 cells expressing Case12 in response to 100 μM ATP.
Images were captured every 0.294 sec on the confocal microscope.
|
|---|
Case12 suitability to generate stably transfected cells has been proven by Marinpharm company.
Available variants and fusions
| Variant | Description | Related vector | Cat.# | Click for image |
|---|
 |
|
Case12-cyto
|
Case12 codon usage is optimized for high expression in mammalian cells [Haas et al., 1996], but it can be successfully expressed in other heterological systems.
When expressed in mammalian cells, this variant is evenly distributed throughout the cytosol and the nucleus.
|
pCase12-cyto*
|
FP991
|
|
Case12-mito
|
Mitochondrial targeting sequence derived from subunit VIII precursor of human cytochrome C oxidase [Rizzuto et al., 1989; Rizzuto et al., 1995] is fused to Case12 N-terminus. When expressed in mammalian cells, this variant is localized in mitochondria.
|
pCase12-mito*
|
FP992
|
|
References:
-
Haas J, Park EC, Seed B.
Codon usage limitation in the expression of HIV-1 envelope glycoprotein.
Curr Biol. 1996; 6 (3):315-24. / pmid: 8805248
-
Nagai T, Sawano A, Park ES, Miyawaki A.
Circularly permuted green fluorescent proteins engineered to sense Ca2+.
Proc Natl Acad Sci U S A. 2001; 98 (6):3197-202. / pmid: 11248055
-
Nakai J, Ohkura M, Imoto K.
A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein.
Nat Biotechnol. 2001; 19 (2):137-41. / pmid: 11175727
-
Rizzuto R, Brini M, Pizzo P, Murgia M, Pozzan T.
Chimeric green fluorescent protein as a tool for visualizing subcellular organelles in living cells.
Curr Biol. 1995; 5 (6):635-42. / pmid: 7552174
-
Rizzuto R, Nakase H, Darras B, Francke U, Fabrizi GM, Mengel T, Walsh F, Kadenbach B, DiMauro S, Schon EA.
A gene specifying subunit VIII of human cytochrome c oxidase is localized to chromosome 11 and is expressed in both muscle and non-muscle tissues.
J Biol Chem. 1989; 264 (18):10595-600. / pmid: 2543673
-
Souslova EA, Belousov VV, Lock J, Stromblad S, Kasparov S, Bolshakov AP, Pinelis VG, Labas YA, Lukyanov S, Mayr LM, Chudakov DM.
Single fluorescent protein-based Ca2+ sensors with increased dynamic range.
BMC Biotechnol. 2007; 7 :37. / pmid: 17603870
|












