
|
Cyan-to-green photoswitchable fluorescent protein PS-CFP2
- Monomer, succesful perfomance in fusions
- Irreversible photoconversion from a cyan to a green fluorescent form
- High contrast of photoconversion
- High pH stability allowing labeling of acidic organelles
- Recommended for tracking cell, organelle, and protein movement, monitoring the protein turnover and superresolution imaging
Performance and use
PS-CFP2 can be easily expressed and detected in a wide range of organisms. Mammalian cells transiently transfected with PS-CFP2 expression vectors display an evenly distributed cyan signal in 12-20 hrs after transfection. No cytotoxic effects are observed.
PS-CFP2 successful performance has been proven in many fusions including that with cytoplasmic β-actin, BH3 interacting domain death agonist (BID), nucleolar protein fibrillarin, and dopamine transporter (hDAT).
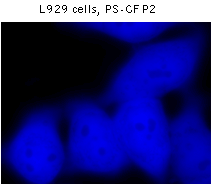 | 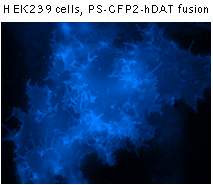 | Fluorescent microscopy of mammalian cells expressing PS-CFP2 protein.
|
|---|
High pH stability: Before photoactivation, PS-CFP2 exhibits a high pH stability with a pKa of 4.3. No changes are observed neither in the shape nor in the amplitude of fluorescence spectra within a pH range of 5.0 and 9.0. This makes it possible to target PS-CFP2 to acidic organelles such as endosomes and lysosomes. After photoswitching, PS-CFP2 has a pKa of 6.1, similar to that of other GFP-like proteins with a phenolate anion chromophore (e.g. EGFP).
High contrast of photoconversion: In response to intense 400 nm light irradiation, PS-CFP2 undergoes irreversible photoconversion expressed in a decrease in cyan fluorescence and appearance of a 490 nm excitation peak with emission maximum at 511 nm. After complete photoconversion, green fluorescence of PS-CFP2 increases more than 400 times, whereas the level of cyan fluorescence drops more than 5.5 times lower. The ratio of green fluorescence of the activated and inactivated form is four times higher for PS-CFP2 than for PA-GFP [Patterson and Lippincott-Schwartz, 2002]. Unlike that of PA-GFP, emission spectrum of PS-CFP2 changes completely, switching from cyan to green fluorescence. Thus, the increase in the green-to-cyan fluorescence ratio accounts for more than a 2000-fold contrast. Considerable decrease of cyan fluorescence during PS-CFP2 photoconversion provides a molecular tool for simultaneous tracking of both the movement of the photoactivated protein and its replacement with the non-activated form.
 | PS-CFP2 photoconversion in transiently transfected mammalian cells (movie).
L929 cells were transiently transfected with a plasmid encoding PS-CFP2 and tested under fluorescent microscope. Before photoswitching no detectable green fluorescence at FITC excitation was seen. In contrast, high-level signal was observed in cyan channel. Upon irradiation with 10-15 micro Joules (about 20-30 W/cm2) violet dye laser (404 nm) for a few seconds a fluorescence increase of more than 300-fold was observed in FITC channel.
|
|---|
Suitability for tracking protein traffic has been demonstrated on example of PS-CFP (the parental variant of PS-CFP2) fused with the human dopamine transporter, hDAT [Chudakov et al., 2004]. PS-CFP-tagged hDAT was expressed in HEK293 cells. As expected, the fusion protein was localized in the cellular membranes. Then PS-CFP-hDAT was selectively photoswitched in the middle parts of two filopodia by short pulse of 404 nm laser irradiation. High contrast of photoconversion allowed monitoring hDAT movement precisely within thin filopodia in the vicinity of a big non-switched PS-CFP-hDAT pool at the filopodia base. At the same time, a decrease in the cyan fluorescence during photoswitching allowed monitoring non-switched PS-CFP-hDAT molecules entering the activated region.
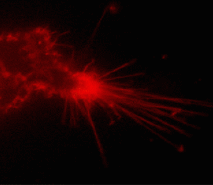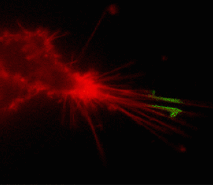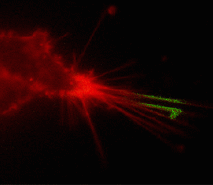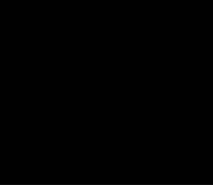 | Tracking PS-CFP-hDAT fusion movement within filopodia during 15 min (movie).
Signals in cyan and FITC channels are shown in red and green pseudocolors, respectively.
|
|---|
When expressed heterologously, hDAT is capable of endocytosis. To test whether early endosomes are able to exchange cargo proteins such as hDAT, PS-CFP-hDAT fusion was selectively photoswitched in several endosomes. Endosomes (both photolabeled and intact) were monitored within the whole cell for more than an hour after the photoactivation. They exhibited fast and rather chaotic intracellular movement. Two endosomes drew together to form a doublet. One of them contained photoswitched PS-CFP-hDAT and soon after their contact PS-CFP-hDAT mutual exchange between the endosomes was occurred: cyan fluorescence of the activated endosome recovered while green fluorescence of the second endosome grew.
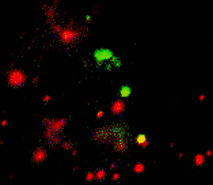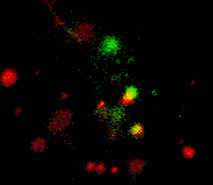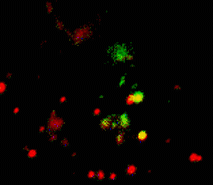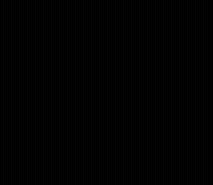 | Tracking of PS-CFP-hDAT fusion within early endosomes for 45 min (movie).
Signals in cyan and FITC channels are shown in red and green pseudocolors, respectively.
|
|---|
Suitability for FRET applications has been demonstated using a fusion construct comprising fluorescent donor (PS-CFP) and acceptor (PhiYFP) proteins with a linker containing Factor Xa protease cleavage site [Souslova and Chudakov, 2006]. In these fusions energy from the excited donor protein migrates partly to the acceptor due to FRET. Incubation of purified fusion constructs with Factor Xa protease eliminates FRET and leads to a gradual increase in the donor emission peak and a simultaneous decrease in the acceptor emission.
Excitation at 402 nm resulted in a substantial yellow fluorescence emission, showing effective FRET. Digestion of the linker between the two fluorescent proteins separated fluorophores and disrupted FRET. Increase in the donor-to-acceptor emission ratio after their separation reached 6.85-fold, which is better than that for any other reported GFP-like protein pairs.
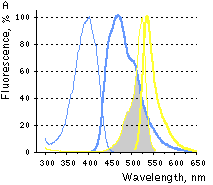 | 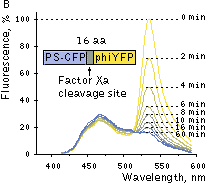 | FRET between PS-CFP and PhiYFP.
(A) Excitation (thin lines) and emission (thick lines) spectra of PS-CFP (blue) and phiYFP (yellow) are shown individually. Spectral overlap is filled with gray. (B) Emission spectra of the PS-CFP-Xa-phiYFP fusion are shown before (yellow) and at various time points after commencing digestion with Factor Xa protease (yellow-blue hues of the spectral lines). When excited at 400 nm the uncleaved construct emitted mainly yellow light that gradually dimmed upon cleavage of the linker.
|
|---|
PS-CFP2 can be used for careful determination of protein half-life as it has been described for Dendra2 in [Zhang et al., 2007]. In the method proposed, cells are transfected with a construct coding for target protein fused with a photoswitchable tag. A steady-state concentration of the fusion protein and corresponding fluorescent signal depends on protein synthesis and maturation rates as well as protein degradation rate. After photoconversion of the photoswitchable tag in a whole cell, a pool of distinct fluorescent molecules appears, which is independent of the synthesis and maturation of the new PAFP molecules. Thus, the decay of the activated fluorescence directly corresponds to the degradation of the PAFP-tagged protein. Time-lapse imaging of the activated signal allows for quantification of degradation process in real-time at the single cell level.
PS-CFP2 as a label for the dual-color superresolution imaging: Photoactivated localization microscopy (PALM) allows imaging of intracellular proteins at nanometer spatial resolution [Betzig et al., 2006]. Now it is possible to perform dual-color superresolution imaging using PS-CFP2 and second, green-to-red photoswitchable protein (like Dendra2) [Shroff et al., 2007]. It has been demonstrated that cyan-to-green photoactivation of PS-CFP2 works perfectly and, importantly, "higher spatial resolution can be obtained with PS-CFP2 than with Dronpa". Dual-color superresolution imaging using PS-CFP2 can reveal the spatial relationship between two proteins in whole fixed cells down to the nanometric level.
References:
-
Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF.
Imaging intracellular fluorescent proteins at nanometer resolution.
Science. 2006; 313 (5793):1642-5. / pmid: 16902090
-
Chudakov DM, Verkhusha VV, Staroverov DB, Souslova EA, Lukyanov S, Lukyanov KA.
Photoswitchable cyan fluorescent protein for protein tracking.
Nat Biotechnol. 2004; 22 (11):1435-9. / pmid: 15502815
-
Patterson GH, Lippincott-Schwartz J.
A photoactivatable GFP for selective photolabeling of proteins and cells.
Science. 2002; 297 (5588):1873-7. / pmid: 12228718
-
Shroff H, Galbraith CG, Galbraith JA, White H, Gillette J, Olenych S, Davidson MW, Betzig E.
Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes.
Proc Natl Acad Sci U S A. 2007; 104 (51):20308-13. / pmid: 18077327
-
Souslova EA, Chudakov DM.
Photoswitchable cyan fluorescent protein as a FRET donor.
Microsc Res Tech. 2006; 69 (3):207-9. / pmid: 16538627
-
Zhang L, Gurskaya NG, Merzlyak EM, Staroverov DB, Mudrik NN, Samarkina ON, Vinokurov LM, Lukyanov S, Lukyanov KA.
Method for real-time monitoring of protein degradation at the single cell level.
Biotechniques. 2007; 42 (4):446, 448, 450. / pmid: 17489230
|












