|
||||||||||
|
||||||||||
SUPPORT/Methods of nucleic acid research:

|
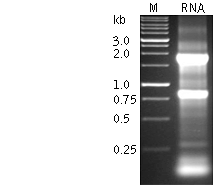
RNA analysis on non-denaturing agarose gel electrophoresis1. The following gel electrophoresis conditions are recommended:
2. Heat an aliquot of the RNA solution at 70°C for 1 min and place it on ice before loading on a gel. 3. Load a known amount of DNA or RNA ladder alongside your RNA sample as a standard for determining the RNA concentration. RNA concentration can be roughly estimated assuming that the efficiency of EtBr incorporation in rRNA is the same as for DNA (the ribosomal RNA may be considered a double-stranded molecule due to its extensive secondary structure). 4. The first sign of RNA degradation on the non-denaturing gel is a slight smear starting from the rRNA bands and extending to the area of shorter fragments. RNA showing this extent of degradation is still good for further procedures. However, if the downward smearing is so pronounced that the rRNA bands do not have a discernible lower edge, this RNA should be discarded. The following characteristics indicate successful RNA preparation:- For mammalian total RNA, two intensive bands should be observed against a light smear. These bands represent 28S and 18S rRNA. The ratio of intensities of these bands should be about 1.5-2.5:1. Intact mammalian poly (A)+ RNA appears as a smear sized from 0.1 to 4-7 (or more) kb with faint 28S and 18S rRNA bands. - In the case of RNA from non-mammalian sources (plants, insects, yeast, amphibians), the normal mRNA smear on the non-denaturing agarose gel may not exceed 2-3 kb. Moreover, the overwhelming majority of invertebrates have 28s rRNA with a so-called "hidden break" (Ishikawa, 1977). In some organisms the interaction between the parts of 28s rRNA is rather weak, so the total RNA preparation exhibits a single 18s-like rRNA band even on a non-denaturing gel. In other species the 28s rRNA is more robust, so it is still visible as a second band. Note: If your experimental RNA is shorter than expected and/or degraded according to electrophoresis data, prepare fresh RNA after checking the quality of RNA purification reagents. If problems persist, you may need to find another source of tissue/cells. In some cases, partially degraded RNA is only available (e.g. tumor samples or hard treated tissues). This RNA can be used for cDNA preparation, however the cDNA sample will contain reduced number of full-length molecules.
- Commonly, genomic DNA contamination does not exceed the amount seen on the agarose/EtBr gel as a weak band of high molecular weight. Such contamination does not affect cDNA synthesis. DNase treatment to degrade genomic DNA is not recommended. In some cases, excess of genomic DNA can be removed by LiCl precipitation or by phenol:chloroform extraction. Nucleic acid research productsReferences
|
|
Copyright 2002-2023 Evrogen. All rights reserved. Evrogen JSC, 16/10 Miklukho-Maklaya str., Moscow, Russia, Tel +7(495)988-4084, Fax +7(495)988-4085, e-mail:evrogen@evrogen.com |