|
||||||||||
|
||||||||||

Case12
SUPPORT |
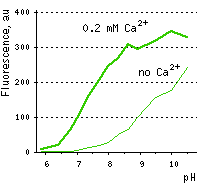
Calcium ion sensor Case12- High dynamic range detection of intracellular Ca2+ level changes Performance and useThe common weak point of conventional calcium sensors is their low pH stability. For example, pKa (meaning of pH at which fluorescence brightness is 50% of maximum) for Pericams reaches as high as 8.0. Therefore, at physiological pH (7.2-7.5) such sensors exhibit low brightness and dynamic range [Nagai et al., 2001]. In contrast, the pKa of Case12 is 7.2 (in the presence of 10 μM Ca2+) close to that reported for G-CaMP [Nakai et al., 2001]. This relatively high pH stability makes Case12 well suitable for in vivo use.
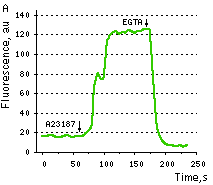
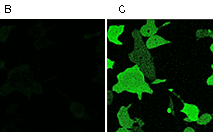
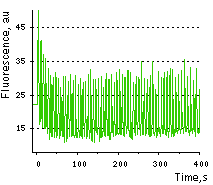
Case12 is characterized by fast maturation at 37°C and bright fluorescent response to Ca2+. It can be directly expressed by target cells, both individually and in fusion with a specific localization signal. No aggregation is observed upon long-term (5 days) expression of Case12 in transiently transfected cells. Monitoring changes in green emission of Case12 in response to intracellular changes of Ca2+ concentration should be carried out by excitation by blue light (488 nm laser line or standard GFP filter set). Emission can be collected at approximately 500-540 nm. Intensity of excitation light should be individually determined for particular biological system and instrumentation. In general, we recommend that you minimize excitation light intensity and duration. Note: Yellow fluorescent core of Case12 undergoes partial photoconversion to a dark state upon irradiation with blue light. It means that an apparent "bleaching" effect occurs at the beginning of time series imaging of cells expressing Case12 protein. Unlike the real bleaching, in the case of Case12, signal drops to the level of dynamic equilibrium between fluorescent and dark state of the chromophore, and then remains stable. Maximum dynamic range in HeLa cells: HeLa cells transfected with Case12 showed relatively weak green fluorescence, which was detected with a Leica microscope DM IRE2, confocal TCS-SP2, objective HCX-PL-APO-63x/1.40-0.60/OIL. Addition of 20 μM calcium ionophore A23187, allowing calcium to enter cells (2 mM Ca2+ in the medium), resultes in 5-6-fold increase in green fluorescence brightness. Subsequent addition of 20 mM EGTA removes Ca2+ and decreases the fluorescence signal close to baseline level, with the final contrast of 11-12-fold. Monitoring of Ca2+ changes under physiological conditions: Mammalian cells expressing Case12 display a nice high dynamic range response upon addition of ATP at a final concentration of 100 μM. This experiment clearly showes that Case12 fluorescence response to Ca2+ oscillations is fast and reversible. It also demonstrates that the sensor responds to changes in Ca2+ concentration in living cells in the nanomolar range.
Case12 suitability to generate stably transfected cells has been proven by Marinpharm company. References:
|
|
Copyright 2002-2023 Evrogen. All rights reserved. Evrogen JSC, 16/10 Miklukho-Maklaya str., Moscow, Russia, Tel +7(495)988-4084, Fax +7(495)988-4085, e-mail:evrogen@evrogen.com |