|
||||||||||
|
||||||||||

ArrestRed
SUPPORT |
|
||||||||||||||||||||||||||||||||||||
|
ArrestRed (the scientific name is H2B-tKR) is a novel optogenetic tool, which can be used to study the roles of specific cell populations in development, regeneration, and carcinogenesis. This is a chimeric protein composed of a tandem version of genetically-encoded photosensitizer KillerRed fused to histone H2B protein [Serebrovskaya et al., 2011]. Expression of ArrestRed in mammalian cells results in correct chromatin labeling and does not interfere with cellular division. The illumination of the ArrestRed expressing cells by intense green light leads to blockage of cell proliferation. The effect of ArrestRed activation lasts for about 24 hours, after that approximately 90% of ArrestRed expressing cells resume division. Repeated light illuminations allow to maintain cells in the non-dividing state for longer periods. The inhibitory effect of ArrestRed on cell cycle progression is attributed to generation of reactive oxygen species (ROS) upon ArrestRed activation [Bulina et al., 2006]. Light-induced generation of ROS leads to massive damage of genomic DNA and activation of repair machinery. In turn, it causes cell cycle checkpoints activation and cell cycle arrest. After successful DNA reparation interphase cells restore normal proliferation. |
Main properties
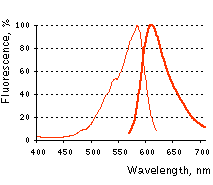
Normalized excitation (thin line) and emission (thick line) spectra. |
| ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Recommended antibodies, filter sets, and activating parameters
ArrestRed can be recognized using Anti-KillerRed antibody (Cat.# AB961) available from Evrogen.
Before light activation, ArrestRed can be detected in cell nuclei using TRITC filter set or similar. Recommended Omega Optical filter sets are QMAX-Red and XF174.
ArrestRed is activated by green-light irradiation at 540-580 nm. Light activation of ArrestRed is accompanied by profound photobleaching (reduction of red fluorescent signal).
For activation of ArrestRed in cell cultures in vitro the Arc-lamp irradiation (540-580 nm, 0.5 W/cm2, 2 min) is strongly recommended; laser-light irradiation in confocal mode is less efficient.
For activation of ArrestRed in the whole organism in vivo the Arc-lamp (illumination through 10x objective, 540-580 nm, 170 mW/cm2, 20 min) or the LED array (525 nm, 45 mW/cm2, 1 hour illumination) can be applied.
The source of irradiation, irradiation time and intensity of green light must be individually determined for particular biological system and instrumentation.
Excessive activation of ArrestRed may result in cell death.
Performance and use
ArrestRed can be used for selective inhibition of cell cycle progression in vitro and in vivo. It can be expressed and activated in various experimental systems, including mammalian cells and Xenopus laevis.
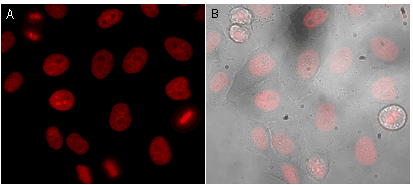
Mammalian cells transiently transfected with ArrestRed expression vector give detectable red fluorescent signals in 24 hours after transfection.
Before light activation, ArrestRed enables correct chromatin labeling and does not interfere with cellular division.
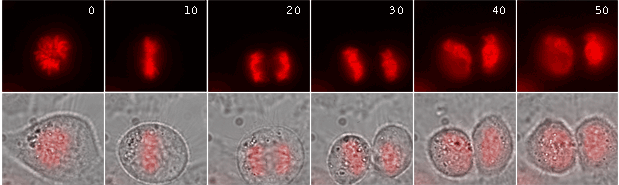 |
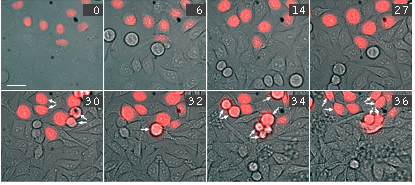
Time-lapse images of a representative single cell undergoing mitosis (numbers indicate time in minutes; red fluorescence and overlay of red fluorescence and transmitted light are shown) |
|---|
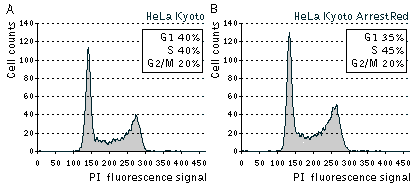 | Cell cycle distribution in asynchronously growing populations of parental HeLa Kyoto and stably transfected HeLa Kyoto ArrestRed cells.Cells were analyzed by flow cytometry after staining with PI (propidium iodide) for the DNA content. |
|---|
Effects of light-activated ArrestRed on cell division in vitro:
Activation of ArrestRed in either transiently or stably transfected HeLa cells results in complete blockage of cell division for about 24 hours. During this time, cell nuclei have interphase morphology, and no cells undergo division. At the same time, most cells remain viable with no membrane blebbing, loss of attachments, cell shrinkage, or other signs of cell death. Over a second 24-h period, (24-48 h after activation of ArrestRed) approximately 90% of the ArrestRed-transfected cells undergo mitosis.
Activation of ArrestRed in mitotic cells results in nondisjunction of chromosomes and the cells are unable to complete division normally. In the end the cells return to interphase morphology with a decondensed tetraploid chromatin.
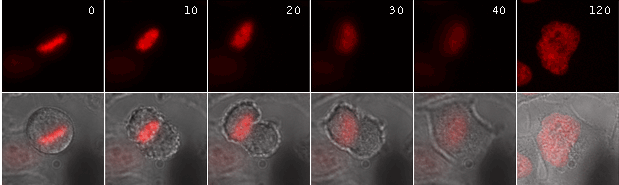 | Time-lapse images of a representative HeLa cell expressing ArrestRed.Activation of ArrestRed was performed during metaphase (numbers indicate time in minutes; red fluorescence and overlay of red fluorescence and transmitted light are shown). |
|---|
Effects of light-activated ArrestRed in vivo:
The effects of light-induced activation of ArrestRed in the whole organism in vivo were demonstrated in transgenic Xenopus laevis embryos [Serebrovskaya et al., 2011].
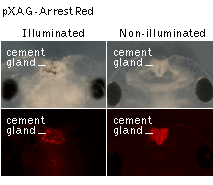
In one set of experiments, the Xag2 promoter was used to specifically direct the ArrestRed expression in the cement gland, a provisory organ located at the rostral end of the embryonic head. Activation of ArrestRed (green light illumination with LED array, 525 nm, 45 mW/cm2, 1 hour) in transgenic embryos at the early neurula stage leads to clear retardation of the cement gland differentiation observed at the tadpole stage.
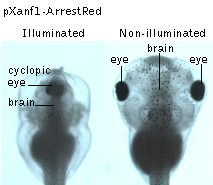
In another set of experiments, the tissue-specific promoter of the homebox gene Xanf1 was used to specifically induce the ArrestRed expression in the cells of the anterior neural fold between the middle gastrula and the late neurula stages of the development. Activation of ArrestRed (green light illumination with LED array, 525 nm, 45 mW/cm2, 1 hour) in transgenic embryos at the early-midneurula stages leads to various degrees of forebrain reduction accompanied by prominent optic stalk dysplasia, which in extreme cases resulted in a complete cyclopic phenotype observed at the tadpole stage.
References:
- Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006; 24 (1):95-9. / pmid: 16369538
- Serebrovskaya EO, Gorodnicheva TV, Ermakova GV, Solovieva EA, Sharonov GV, Zagaynova EV, Chudakov DM, Lukyanov S, Zaraisky AG, Lukyanov KA. Light-induced blockage of cell division with a chromatin-targeted phototoxic fluorescent protein. Biochem J. 2011; 435 (1):65-71. doi: 10.1042/BJ20101217 / pmid: 21214518
|
Copyright 2002-2023 Evrogen. All rights reserved. Evrogen JSC, 16/10 Miklukho-Maklaya str., Moscow, Russia, Tel +7(495)988-4084, Fax +7(495)988-4085, e-mail:evrogen@evrogen.com |