
|
Genetically-encoded photosensitizer KillerOrange
- Blue and green light-induced production of reactive oxygen species
- Direct expression in cells, easy targeting to various subcellular compartments
- No exogenous chemical compounds required for chromophore maturation
- Not toxic before activation by blue or green light irradiation
- Recommended for selective light-induced protein inactivation and cell killing
Performance and use
KillerOrange can be used for in vivo killing of selected cells. It can be expressed and induced in various experimental systems, including bacteria and mammalian cells.
KillerOrange's suitability for light-induced killing of prokaryotic cells has been demonstrated using E. coli XL1-Blue strain:
Aliquots of E. coli XL1 Blue cells transformed with vectors expressing EGFP (non-phototoxic control), KillerOrange (KO) and KillerRed (KR) were mixed together. The E. coli cells used for the experiment were incubated overnight at 4°C to increase the fraction of the mature protein. The suspension was than diluted to the optical density of 0.05 and aliquoted into transparent PCR tubes (40 μl of the suspension per tube). Samples of EGFP-KO-KR mixture were illuminated with one of LEDs of X-Cite XLED1 illuminator (Lumen Dynamics Group Inc.) The LEDs named "BDX" (450–495 nm), "BGX" (505-545 nm) and "GYX" (540-600 nm) were used. The power of all LEDs was set to be about 80 mW/cm2. All aliquots were then spread over the Petri dishes, cultivated for 12-16 hours at 37°C, washed out and analyzed with fluorescence-activated cell sorter (FACS Aria III, BD Biosciences).
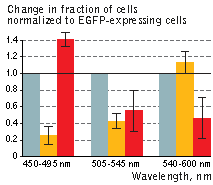 | KillerOrange (KO) and KillerRed (KR) toxicity under light illumination at different wavelengths.
Illumination with 540-600 nm light resulted in the selective removal of KillerRed-expressing cells, while the illumination with 450-495 nm light killed the majority of KillerOrange-expressing cells. Interestingly, 505–545 nm light illumination was almost equally efficient in killing both KillerOrange and KillerRed cells. Thus, by combining different light sources one can achieve precise control over cell populations expressing KillerOrange and KillerRed.
Grey column – EGFP, orange column – KillerOrange, red column – KillerRed. Error bars represent SD, N=4.
|
|---|
KillerOrange-mediated killing of eukaryotic cells:
HEK 293 cells were grown in DMEM medium on 6-cm Petri dishes until about 70% confluency. They were then transiently transfected using FuGene HD (Roche) with one of the following plasmids: pKillerRed-dMito, pKillerOrange-dMito (Evrogen, Cat.# FP224) or pEGFP-N1 (Clontech). 24 hours after transfection cells were washed off from the dish, centrifuged, resuspended in PBS pH7.4 and mixed in pairs (KillerOrange with EGFP, KillerRed with EGFP) in polystyrene optical cuvettes (Sarstedt). The suspensions were then split in two halves, first one was illuminated with custom LEDs while keeping the second in the darkness for the same period of time. For blue light illumination, assembly of 7 Luxeon Star LXML-PR01-0500 Rebel LEDs, Lumileds (dominant wavelength is 447 nm, spectral half-width is 20 nm), for orange illumination we used 7 Luxeon Star 7 LXML-PL01-0040 Rebel LEDs, Lumileds (dominant wavelength is 590 nm, spectral half-width is 20 nm). The light power at the sample was set to be about 60 mW for 447 nm light and about 20 mW for 590 nm light. After illumination the cells were seeded back to Petri dishes, incubated for 24 hours, washed off again, and analyzed cell fluorescence with flow cytometer (Beckman Coulter, USA).
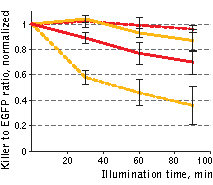 | Phototoxicity of mitochondria-targeted versions of KillerOrange and KillerRed in mammalian cells.
KillerOrange, KillerRed and EGFP-expressing cells were mixed and illuminated with 477 nm or 590 nm light. Y-axis depicts changes in fractions of KillerOrange (yellow lines) or KillerRed-expressing (red lines) cells in the population. Cell fractions were normalized to the fractions in non-illuminated sample.
Red dashed line – KillerRed, 447 nm; orange dashed line – KillerOrange, 447 nm; red line – KillerRed, 590 nm; orange line – KillerOrange, 590 nm. Error bars represent SD, N=3.
|
|---|
A strong decrease in the fraction of KillerRed-expressing cells was seen in the population that passed through the orange light irradiation. In contrast, KillerOrange-expressing cells were substantially removed from the population of cells that was illuminated with the blue light. Taken together, these results show that KillerOrange is phototoxic to mammalian cells and that KillerRed and KillerOrange can be used simultaneously to independently control fates of two cell populations.
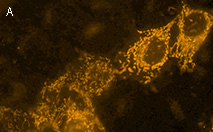 | 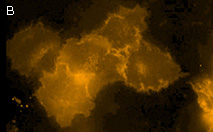 | KillerOrange expression in mammalian cells.
(A) Transiently transfected HeLa cells expressing mitochondria-targeted KillerOrange.
(B) Transiently transfected HeLa cells expressing membrane-targeted KillerOrange.
|
|









